Test post
Abstract
Rigenera® is a novel class-1 medical device The manufacturer’s protocol has been supported for a wide variety of clinical uses in the field of regenerative medicine. This study aimed to evaluate its potential use for in vitro cell models. Human primary oral fibroblasts were cultured under standard conditions and processed through Rigenera® over a time course of up to 5 min. Cell viability was assessed using a Trypan Blue exclusion test. It is possible to process fibroblasts through Rigenera® although an initial reduction of cell viability was observed. Additionally, debris was evident in the cell suspension of the processed samples. Scanning electron microscopy (SEM) microanalysis of the debris and electron energy-loss spectroscopy confirmed the presence of metal wear possibly due to the processing conditions used in this study. Interestingly, pore sizes within Rigeneracons® grids were found to range between 250–400 μm. This is the first report assessing the suitability of Rigenera® and Rigeneracons® for in vitro applications. Whilst Rigenera® workflow was found to be amenable to laboratory uses, our results strongly suggest that further research and development is necessary to support the utilization of this technology for enrichment of micro-graft derived cells and cell sorting in vitro.
1. Introduction
Research in the field of stem cells and their potential application in regenerative medicine have captured attention in the 21st century. The physiological process of tissue regeneration is fundamental for the maintenance of tissues and organs throughout the human body. The importance of stem cells lies in their ability to differentiate into various cell types for the purpose of tissue regeneration.
One promising source of stem cells are those derived from the epidermis [1], including that of the oral mucosa, such as oral keratinocytes and fibroblasts [2,3]. Additionally, the dental pulp has been shown to be a rich source of stem cells [4].
Fibroblasts are abundant within the connective tissue where they maintain the structural integrity of connective tissue by synthesizing and remodelling components of the extracellular matrix and regulate autoimmunity, organ development, wound healing, inflammation and fibrosis [3,5]. Furthermore, fibroblasts exhibit many properties similar to mesenchymal stem cells (MSCs), being both plastic adherent and multipotent cells [3]. The multipotent characteristics of fibroblasts have sparked interest in their use in the field of regenerative medicine.
Although the isolation of stem cells can be achieved, therapeutic application remains a challenge [6]. A major shortcoming that continues to hinder the translation of
stem cell research from bench to bedside is the ex vivo manipulation of donor tissues, a necessary step to isolate progenitor cells. This is currently achieved using chemical agents. In 2006, Human Brain Wave (HBW) designed Rigenera®, a chairside technology introduced as an innovative technique that allows isolation of stem cells from autologous micro-grafts for application in clinical regenerative procedures. Using the Rigenera® protocol, in just one surgical procedure, the patient is both donor and recipient of calibrated micro-grafts. This takes place within a single procedural time and is is capable of enriching micro-graft-derived progenitors cells for regeneration within the recipient site [7]. Importantly, this technique is based on a principle of mechanical size-selection, thus reducing the risk of contamination or alteration of the sample. At present, Rigenera® is an innovative and promising application in regenerative medicine, particularly in the fields of dental surgery, dermatology, plastic surgery, maxilla-facial surgery, orthopaedics, aesthetic medicine, and wound healing [7–16].
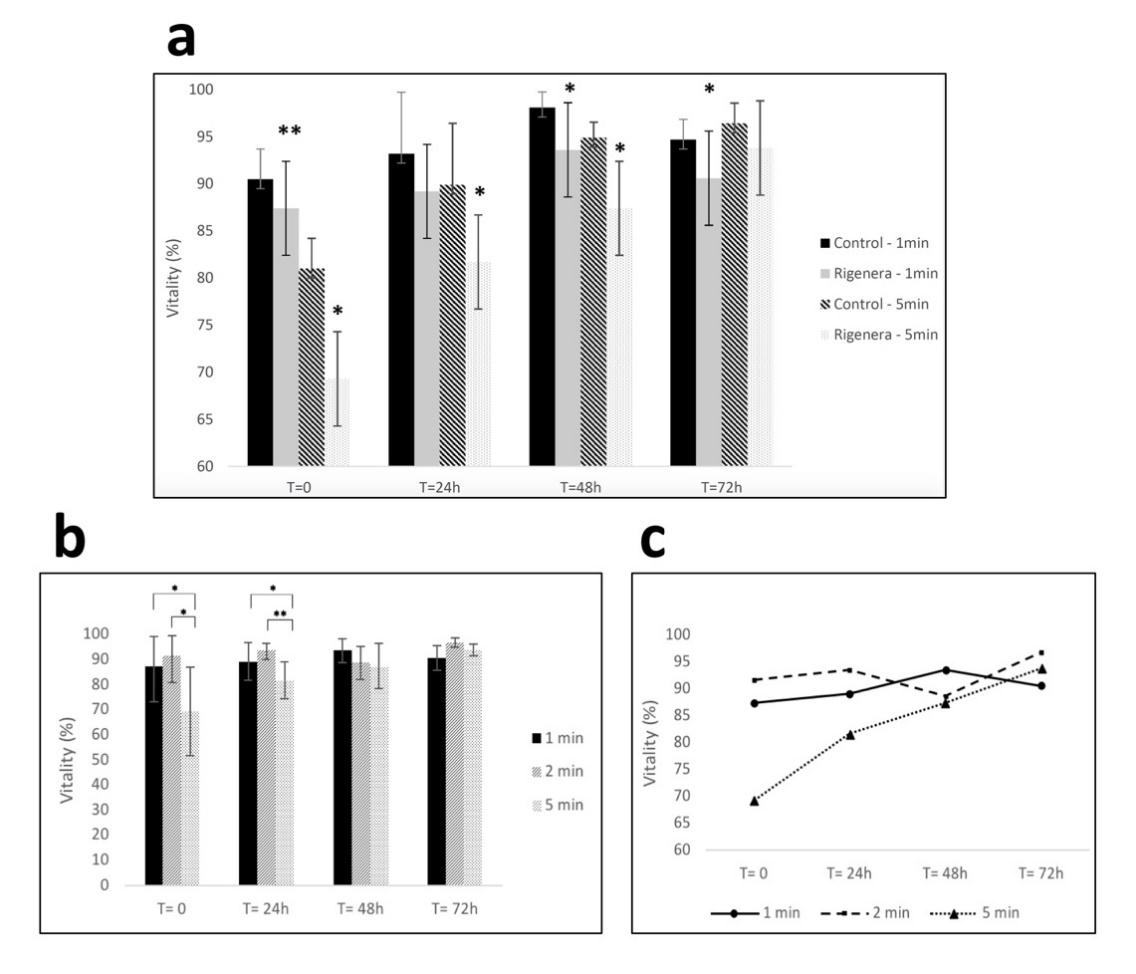
Figure 1. (a) Fibroblast viability for control and samples processed with Rigenera® for 1-min and 5-min. Significant differences in viability between control and processed cells are indicated as * = p < 0.05 or ** p < 0.01. (b,c) Viability of fibroblasts at 0/24/48/72 h time points after being processed with Rigenera® for 1, 2 and 5 min. Statistical significance is given as follows: * p < 0.05; ** p < 0.01.
3.2. Rigenera® Protocol is Associated with the Release of Metal Particles
Presence of opaque debris was visualized during experiments in all fibroblasts samples processed with Rigenera® (Figure 2). In detail, when inspected under inverted light microscopy, all samples regardless of processing duration presented with heterogenous debris ranging in size from 3–400 μm. The amount of debris produced was also found within the cell-free control, which consisted of only UltraPure™ distilled water (Invitrogen) for one minute (Figures 2a and 3a). The debris collected showed random formation of aggregates in various sizes ranging from 0.1 μm to about 400 μm in diameter. EELS microanalysis of the debris revealed mainly metallic and minute plastic constituents. Elemental point-by-point analysis of the multiple debris particles revealed values ranging between 36.64%–52.96% iron, 7.45%–9.31% chromium, 37.28%–37.72% carbon and about 1% silica by weight (Figure 3b–e).
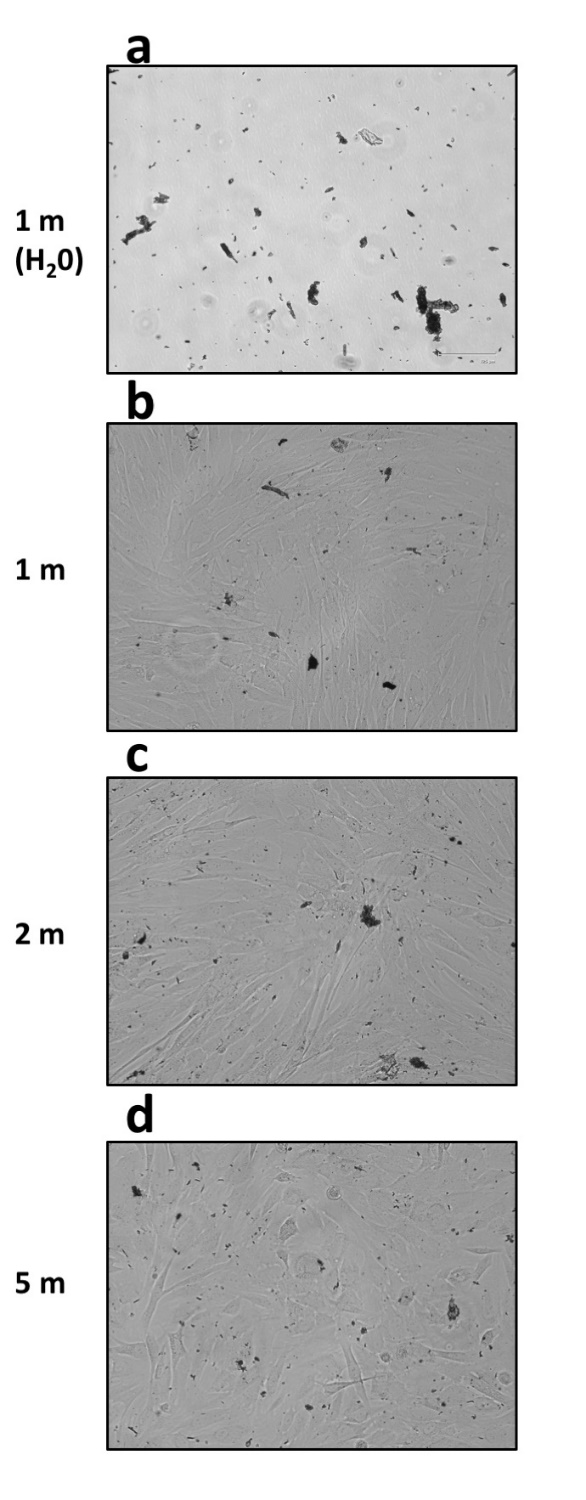
Figure 2. Presence of abundant and heterogenous debris observed at inverted light microscopy in samples processed with Rigenera® (EVOS FLoid Cell Imaging Station, ×460 optical magnification). (a) The cell-free control sample, which consisted of UltraPure™ distilled water (10977023, Invitrogen) alone processed for 1 min. Fibroblast samples were processed instead for 1, 2, or 5 min ((b–d) respectively).
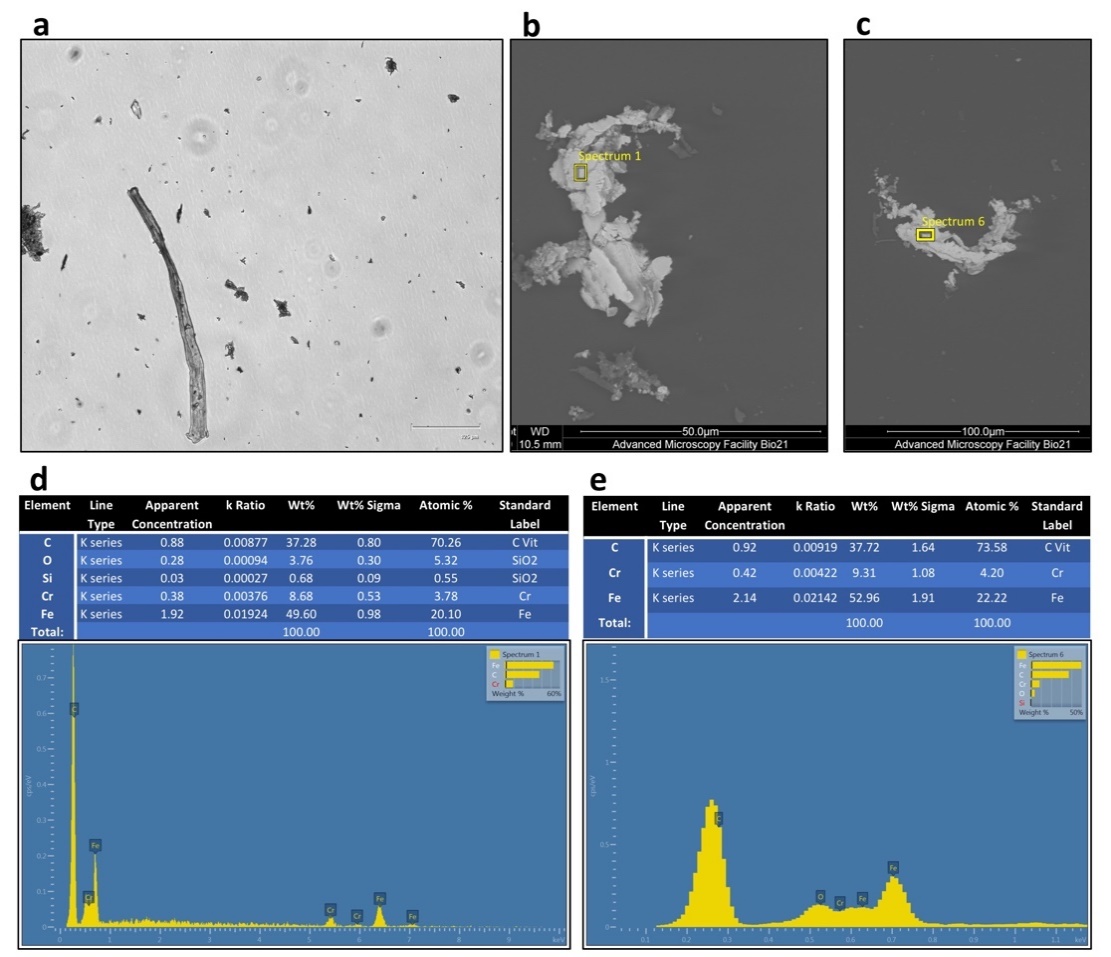
Figure 3. Aspect of the cell-free control sample (UltraPure™ distilled water alone) processed for 1 min at inverted light microscopy (a) and scanning electron microscopy (SEM) (FEI Tecnai F30) (b,c). Compositional microanalysis via electron energy-loss spectroscopy (EELS) of the debris particles identified in panel b (d) and c (e) respectively. The debris was metallic in nature with primarily iron, carbon, and chromium as its elemental composition by weight. Minute concentrations of Silica was detected.
3.3. Rigeneracons® Internal Components Show Marked Degree of Wear after Use
The Rigeneracons® disposable devices were carefully disassembled in a biosafety cabinet after use, and the internal metal components, blades and grids, were analysed under reflected light microscopy and SEM. As expected, macroscopic scratch marks were found on the cutting blades (Figure 4), with the gross size of scratch marks found to be greatest after 5 min of processing. Thus, the longer the Rigeneracons® were processed, the more scratch marks were observed. No visible scratch marks were present on the unused blade when investigated under reflected light microscopy. Subsequently, the metal blades of Rigeneracons® were analysed using SEM. Scans of the blades utilised in the 2 and 5 min processing durations showed significant surface roughness, consistent with signs of wear, on the front cutting portion of the blades with visible peeling of the edges (Figure 5a) whereas this was not observed in the unused blade. Debris, forming into aggregates of up to 400 μm, was also found on the blades’ surface (Figure 5b–d).
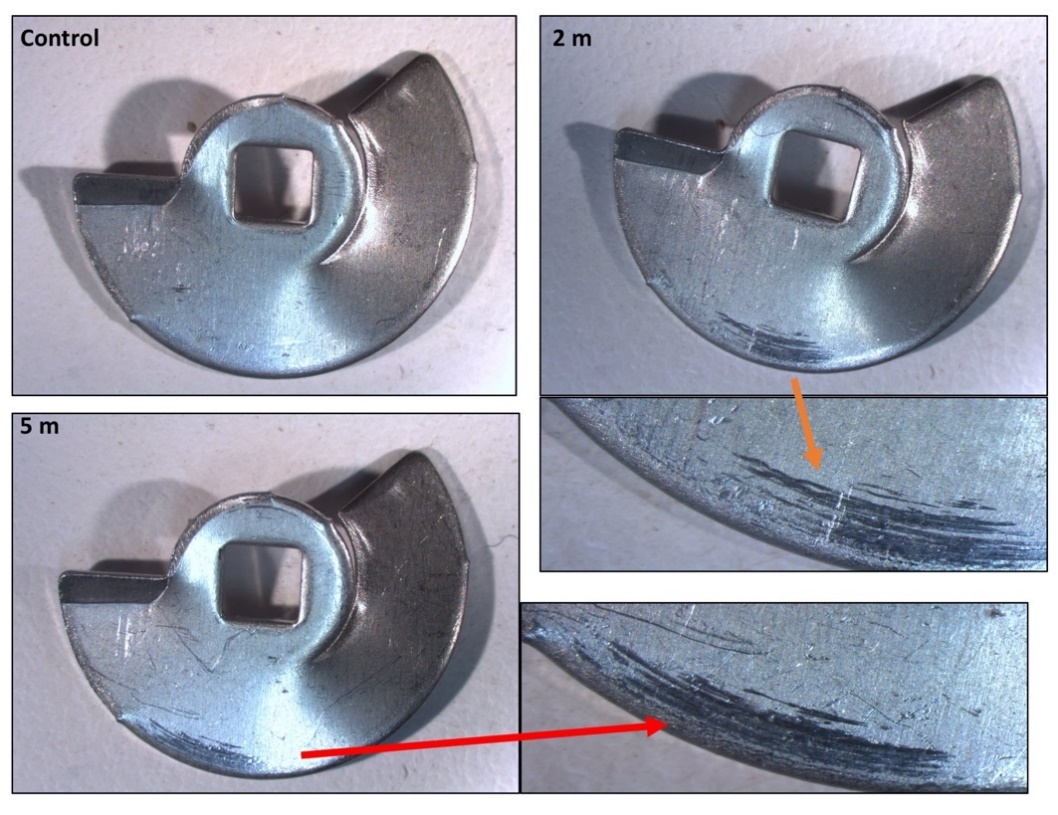
Figure 4. Reflected light microscopy of the Rigeneracons® internal blade when unused (control), after 2 min of processing and after 5-min of processing. The unused blade showed no substantial scratch marks, however, as time of processing was increased, the scratch marks present on the outer rim increased in gross size (greatest after 5 min).
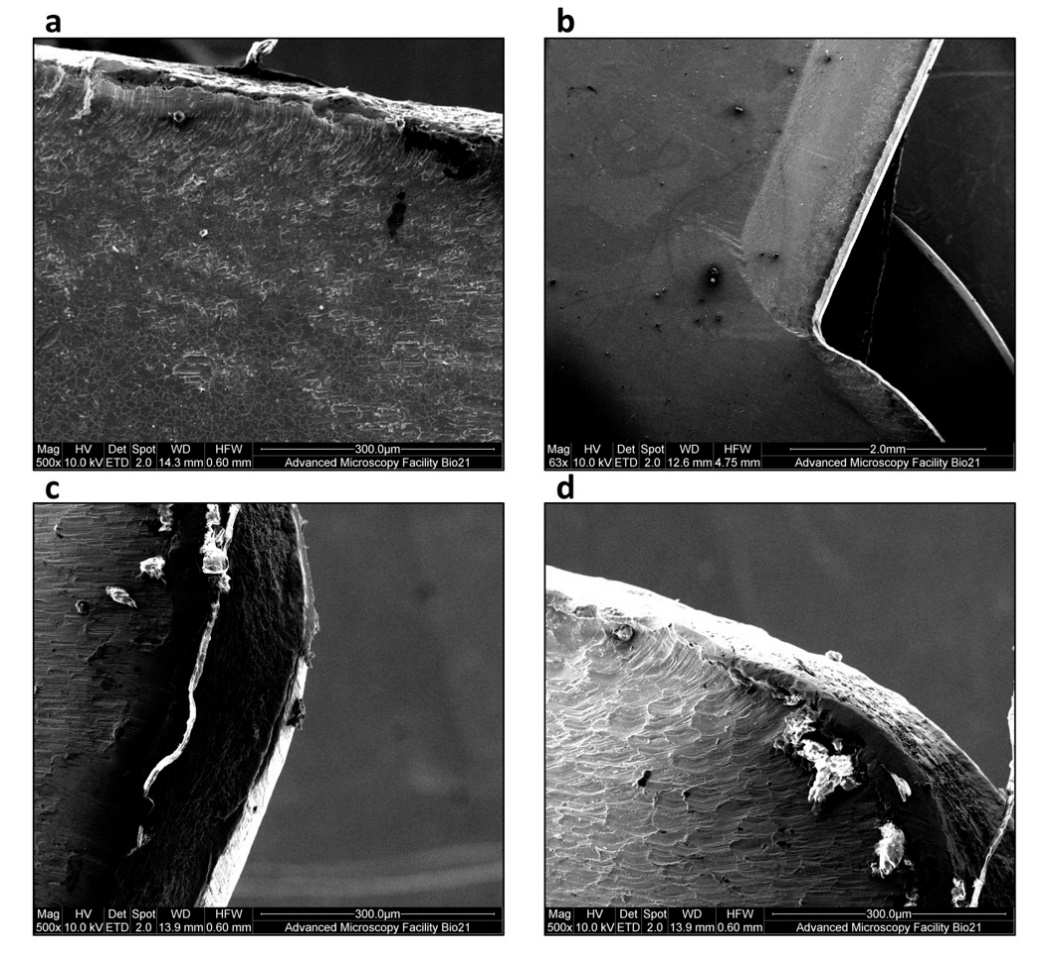
Figure 5. Scanning electron microscope (SEM) observation of blades in Rigeneracons®. A blade after 2 min of processing shows evidence of (a) significant wear as damage on the front cutting portion of the blades with visible peeling of the edges. Debris, forming into aggregates of up to 400 μm, was also found on the blades’ surface (b–d).
3.4. Assessment of Pore Size Range
Given that the metal particle size was larger than the grid’s described pore size (50 μm in diameter), the micromorphology of Rigeneracons® grid was inspected. The metallic grids containing the pores designed for individual cell separation based on cell size were analysed using reflected light microscopy. The damaged portions were the cutting edges of the hexagonal pores. The tips of the cutting surfaces appeared to increase in damage proportional to processing time (Figure 6). These findings were confirmed with SEM observation (Figure 7). Abundant debris was additionally detected (Figure 7b). We further observed that the pores size was significantly different from the described diameter of 50 μm. Pore diameter size was found to range between 250–400 μm, with an average of about 300 μm (Figure 7c,d). The pore size was found to be similar when comparing unused (Figure 7a) and used grids.
Collectively, these data show that the Rigenera® workflow is accompanied by the production of microscopic metal debris. Furthermore, the grid pores are larger than it was expected.
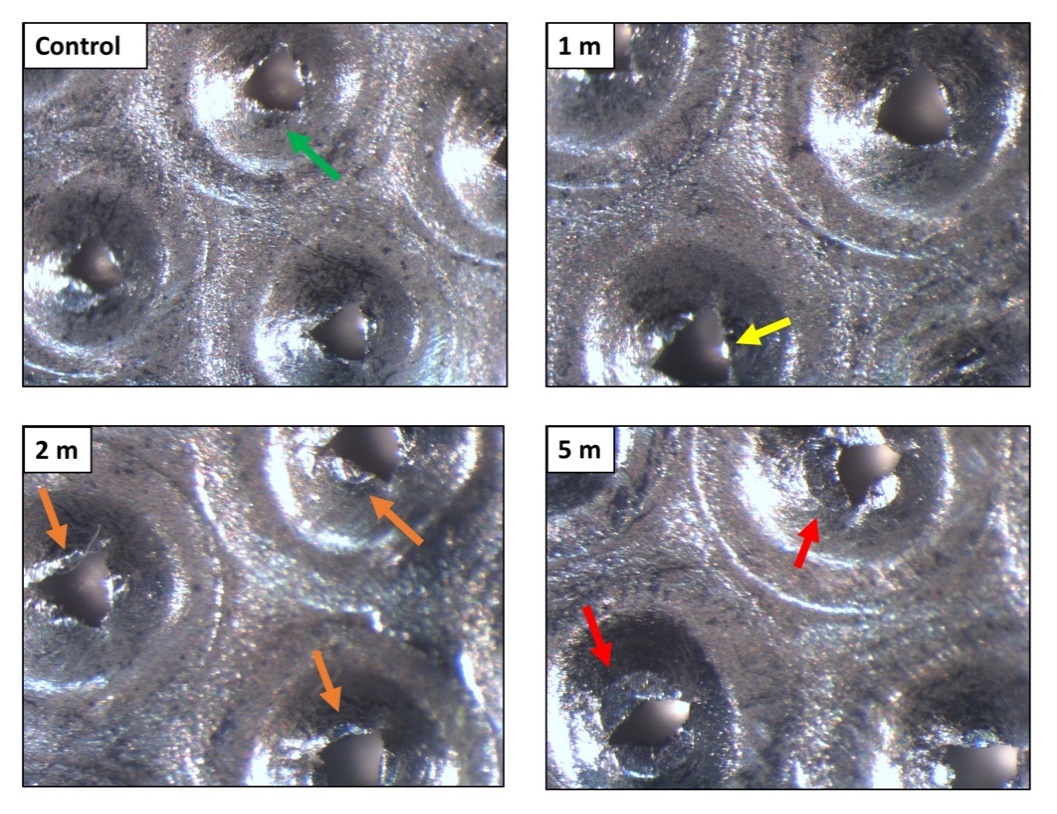
Figure 6. Reflected light microscopy of the Rigeneracons® internal metallic grid when unused (control), after 1 min, 2 min and after 5 min of processing. The control grid showed no damage of the cutting edges of the hexagonal pores (green arrow). The tips of the cutting surfaces appeared to increase in damage proportional to processing time. The damage ranged from mild wear (yellow arrow), medium wear (orange arrows), up to a complete loss of the cutting edges of the hexagonal pores (red arrows).
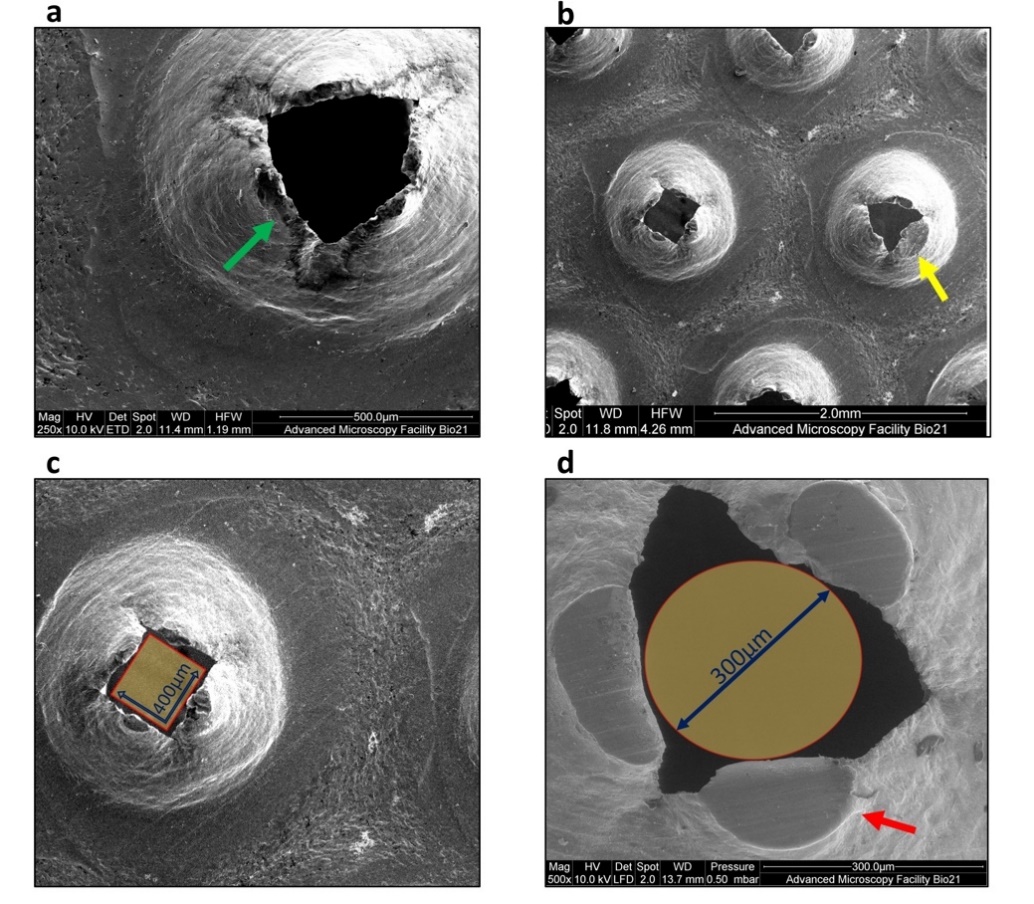
Figure 7. Scanning electron microscope (SEM) observation of the Rigeneracons® steel grid pores. (a) Intact cutting edges of the hexagonal pores (green arrow) in unused disposable device. (b) Spread debris was visible after 2 min of processing together with substantial damage of the cutting edges (yellow arrow). Reference scale measurements show the size of random pores after 5 min of processing. Some of the pores measure just slightly over 400 μm in diameter at the widest span (c) and the majority of them allowed the passage of particles with 300 μm diameter (d).
4. Discussion
This study demonstrates that the Rigenera® protocol can be used in a laboratory setting. However, when applied in vitro, the cell processing determines a significant but transient decrease in viability of cultured fibroblasts. Cells were processed for 5 min and 30% decrease in initial viability was observed. The processing of cells with Rigenera® additionally resulted in samples containing debris. The debris was evident in all samples regardless of processing time and appeared to be metal components of the Rigeneracons®.
The effect on cell viability when processed using Rigenera® is important for its potential in vitro use, particularly in comparison to current cell-sorting techniques such as fluorescence-activated cell sorting (FACS) and magnet-activated cell sorting (MACS), which are proven to maintain cell vitality [21,22]. Therefore, it is important when the Rigenera® protocol is used as an alternative to conventional cell sorting methods in vitro that the vitality is maintained at comparable levels.
In our experimental conditions, the longer the processing time the greater degree of vitality loss, i.e